
Chemistry, 28.11.2019 06:31, emilyswinge4421
When fe(s) reacts with cl2(g) to form fecl3(s) , 400 kj of energy are evolved for each mole of fe(s) that reacts. write a balanced thermochemical equation for the reaction with an energy term in kj as part of the equation.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, bartfrank447
Joseph has hypothesized that sound travels in waves. if he were following the scientific method, what should he do next? a. ask a question. b. test the hypothesis. c. study the results. d. tell other scientists about his hypothesis.
Answers: 1

Chemistry, 22.06.2019 08:00, gomezyonathan93
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

Chemistry, 22.06.2019 11:30, charles8527
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2
Do you know the correct answer?
When fe(s) reacts with cl2(g) to form fecl3(s) , 400 kj of energy are evolved for each mole of fe(s)...
Questions in other subjects:

Mathematics, 24.03.2021 07:30


Mathematics, 24.03.2021 07:30

English, 24.03.2021 07:30

History, 24.03.2021 07:30


Biology, 24.03.2021 07:30

Chemistry, 24.03.2021 07:30



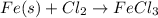
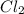 by 2 on the reactant side. Also, we multiply
by 2 on the reactant side. Also, we multiply 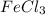 by 2 on product side.
by 2 on product side.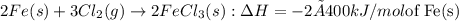
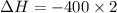 = -800 kJ
= -800 kJ



