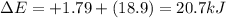Chemistry, 28.11.2019 06:31, SophieStar15
What is δe in kj for a system that receives 1.79 kj of heat from surroundings and has 4.51 kcal of work done on it at the same time. 1 cal = 4.184 j.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, jadepotts3965
Calculate the change in entropy if br2(l) is converted into gaseous br atoms. s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 2

Chemistry, 23.06.2019 00:00, juliannasl
How is the way a mixture is combined different from how a compound is combined?
Answers: 3

Chemistry, 23.06.2019 01:00, Zachgrainger4436
You wish to prepare a buffer consisting of acetic acid and sodium acetate with a total acetic acetate plus acetate concentration of 250 mm and a ph of 5. what concentrations of acetic acid and sodium acetate should you use
Answers: 1
Do you know the correct answer?
What is δe in kj for a system that receives 1.79 kj of heat from surroundings and has 4.51 kcal of w...
Questions in other subjects:



English, 01.01.2020 18:31


History, 01.01.2020 18:31

Mathematics, 01.01.2020 18:31

Mathematics, 01.01.2020 18:31

Biology, 01.01.2020 18:31


History, 01.01.2020 18:31


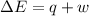
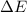 =Change in internal energy
=Change in internal energy
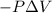 {Work is done on the system is positive as the final volume is lesser than initial volume}
{Work is done on the system is positive as the final volume is lesser than initial volume}
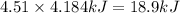 (1kcal = 4.184kJ)
(1kcal = 4.184kJ)