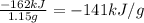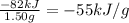Chemistry, 28.11.2019 05:31, bargasdevon123
It has been suggested that hydrogen gas obtained by the decomposition of water might be a substitute for natural gas (principally methane). to compare the energies of combustion of these fuels, the following experiment was carried out using a bomb calorimeter with a heat capacity of 11.3 kj/oc. when a 1.50 g sample of methane gas was burned with excess oxygen in the calorimeter, the temperature increased by 7.3oc. when a 1.15 g sample of hydrogen gas was burned with excess oxygen, the temperature increase was 14.3oc. compare the energies of combustion (per gram) for hydrogen and methane.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, britotellerialuis
Ibeg i need 20. a reaction produces 4.93 l of oxygen, but was supposed to produce 1 mol of oxygen. what is the percent yield?
Answers: 1


Chemistry, 22.06.2019 12:00, KKHeffner02
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 22.06.2019 12:00, winterblanco
What is the lowest number energy level where a d sublevel is found
Answers: 1
Do you know the correct answer?
It has been suggested that hydrogen gas obtained by the decomposition of water might be a substitute...
Questions in other subjects:

Arts, 21.10.2020 19:01

Mathematics, 21.10.2020 19:01




Mathematics, 21.10.2020 19:01


Geography, 21.10.2020 19:01


Mathematics, 21.10.2020 19:01