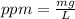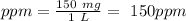Chemistry, 28.11.2019 05:31, jellyangie1
Astock solution contains a mixture of ~100 ppm chloride, fluoride, nitrite, bromide, nitrate and phosphate anions. in order to prepare 1 l of 100 ppm nitrite stock solution, you weigh out 150.0 mg of nano2. the actual concentration of nitrite would be:

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, lemonsalt9378
14. complete and balance the equations for the single displacement reactions. a. zn + pb(no3)2 -> b. al + niso4 -> 15. complete and balance the equations for the double displacement reactions. a. agno3(aq) + nacl(aq) -> b. mg(no3)2(aq) + koh(aq) -> 16. complete and balance the equations for the combustion reactions. a. __ ch4 + o2 -> b. __ c3h6 + o2 -> c. + o2 ->
Answers: 2

Chemistry, 22.06.2019 09:00, Ezekielcassese
What is the percentage composition of carbon in the compound ch4
Answers: 1

Chemistry, 22.06.2019 18:00, Jazmineboo7709
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2

Chemistry, 23.06.2019 00:00, glocurlsprinces
(04.05 hc) analyze the given diagram of the carbon cycle below. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 3
Do you know the correct answer?
Astock solution contains a mixture of ~100 ppm chloride, fluoride, nitrite, bromide, nitrate and pho...
Questions in other subjects:



Mathematics, 23.10.2019 08:50



English, 23.10.2019 08:50

History, 23.10.2019 08:50


German, 23.10.2019 08:50


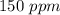
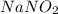 , so:
, so: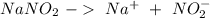
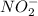 is 1:1
is 1:1