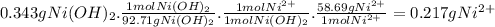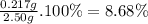To measure the amount of nickel in some industrial waste fluid, an analytical chemist adds 0.110 m sodium hydroxide (naoh) solution to a 25.0 g sample of the fluid and collects the solid nickel(i) hydroxide (ni (oh2) product. when no more ni(oh)2 is produced, he filters, washes and weighs it, and finds that 343. mg has been produced the balanced chemical equation for the reaction is: ni2+(aq) + 2naoh(aq) ni(oh)2(s) + 2 na. (ag) r precipitation | | o acid-base o redox og dx10 ar what kind of reaction is this? if you said this was a precipitation reaction, enter the chemical formula of the precipitate. if you said this was an acid-base reaction, enter the chemical formula of the reactant that is acting as the base. if you said this was a redox reaction, enter the chemical symbol of the element that is oxidized. calculate the mass percent of ni in the sample. be sure your answer has the correct number of significant digits.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, brookemcelhaney
Which of the following methods uses the decay of atomic particles in an object to find its exact age? a. fossil dating b. geologic dating c. radioactive dating d. relative dating
Answers: 1

Chemistry, 22.06.2019 06:00, rigobertogarza2
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2


Chemistry, 23.06.2019 02:20, alejandraluna95
Why dose heating increase the speed at which a solution dissolved in water
Answers: 1
Do you know the correct answer?
To measure the amount of nickel in some industrial waste fluid, an analytical chemist adds 0.110 m s...
Questions in other subjects:



Mathematics, 15.12.2020 21:50

Physics, 15.12.2020 21:50


Biology, 15.12.2020 21:50



Physics, 15.12.2020 21:50

Spanish, 15.12.2020 21:50