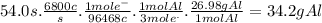Chemistry, 28.11.2019 00:31, angeljaylyn123
In the hall-heroult process, a large electric current is passed through a solution of aluminum oxide (a12o 3) dissolved in molten cryolite (na3aif6) ,resulting in the reduction of the ai�3 to pure aluminum. suppose a current of 6800.a is passed through a hall-heroult cell for 54.0 seconds. calculate the mass of pure aluminum produced. be sure your answer has a unit symbol and the correct number of significant digits.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:40, MathChic68
It is important to wear proper protective equipment in lab even when not actively performing experiments because accidents can affect any researcher, even one not working on an experiment. select the best answer from the choices provided
Answers: 3

Chemistry, 22.06.2019 05:40, timmonskids6027
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 21:00, agarcia24101993
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1

Chemistry, 23.06.2019 00:00, samangelzrose3576
Exit what is the density of an object having a mass of 5.0 g and a volume of 45.0 cm3?
Answers: 1
Do you know the correct answer?
In the hall-heroult process, a large electric current is passed through a solution of aluminum oxide...
Questions in other subjects:

Mathematics, 15.12.2020 21:40

Computers and Technology, 15.12.2020 21:40

Mathematics, 15.12.2020 21:40


Business, 15.12.2020 21:40