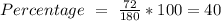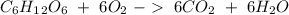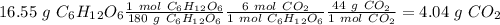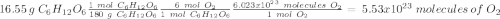Glucose (c6h12o6) is a key nutrient for generating chemical potential energy in biological systems. we were provided 16.55 g of glucose. calculate:
a) the mass percent of carbon in glucose.
b) the mass of co2 produced by the combustion of 16.55 g glucose with sufficient oxygen gas.
c) how many oxygen molecules needed for the completely combustion of 16.55 g glucose?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, amandasantiago2001
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3

Chemistry, 22.06.2019 04:00, lucasrandall
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2


Chemistry, 22.06.2019 20:00, bbyitskeke7160
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3
Do you know the correct answer?
Glucose (c6h12o6) is a key nutrient for generating chemical potential energy in biological systems....
Questions in other subjects:


Mathematics, 26.02.2021 14:00


Business, 26.02.2021 14:00


Mathematics, 26.02.2021 14:00

Mathematics, 26.02.2021 14:00

Chemistry, 26.02.2021 14:00


Mathematics, 26.02.2021 14:00


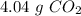
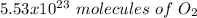
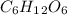 , so the first step is to find the atomic mass of each atom and multiply by the amount of atoms in the molecule.
, so the first step is to find the atomic mass of each atom and multiply by the amount of atoms in the molecule.