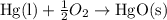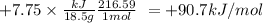Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:50, ddmoorehouseov75lc
If a substance is not at its melting or boiling point, as the heat content of a sample of matter increases, its temperature increases the number of intermolecular bonds decreases the space between particles increases the particles move faster
Answers: 2

Chemistry, 22.06.2019 07:00, coolkid2041
Calculate the number of moles of ethane in 100 grams
Answers: 3


Chemistry, 22.06.2019 17:30, nijanicole164
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2
Do you know the correct answer?
When 18.5 g of hgo(s) is decomposed to form hg(l) and o2(g), 7.75 kj of heat is absorbed at standard...
Questions in other subjects:




Law, 30.11.2020 21:40



Mathematics, 30.11.2020 21:40

Mathematics, 30.11.2020 21:40