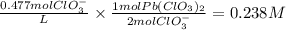Chemistry, 27.11.2019 22:31, astultz309459
A24.00 ml sample of a solution of pb(clo3)2 was diluted with water to 52.00 ml. a 17.00 ml sample of the dilute solution was found to contain 0.220 m clo3−(aq). what was the concentration of pb(clo3)2 in the original undiluted solution? 3.60 × 10−2 m 7.19 × 10−2 m 0.238 m 0.156 m 0.477 m

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, babygirl2984
There is an area in idaho named craters of the moon where most of the ground is covered with basalt, adark gray, igneous rock with no visibl crystals. what can you infer about the geographical history of the area?
Answers: 1

Chemistry, 22.06.2019 20:10, jakhunter354
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3

Chemistry, 22.06.2019 23:30, billybob8514
To find the work done, the force exerted and distance moved are multiplied. a couch is moved twice before you are happy with its placement. the same force was used to move the couch both times. if more work is done the first time it is moved, what do you know about the distance it was moved? a) when more work was done, the couch was moved the same distance. b) when more work was done, the couch was moved less. c) when more work was done, the couch was moved further. d) when more work was done, the couch wasn't moved at all.
Answers: 1
Do you know the correct answer?
A24.00 ml sample of a solution of pb(clo3)2 was diluted with water to 52.00 ml. a 17.00 ml sample of...
Questions in other subjects:

Mathematics, 23.09.2020 18:01






History, 23.09.2020 18:01



Mathematics, 23.09.2020 18:01