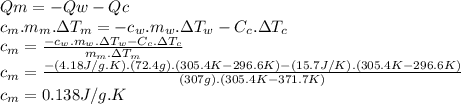Chemistry, 27.11.2019 20:31, cindyc1103
307 g of an unknown mineral is heated to 98.7 °c and placed into a calorimeter that contains 72.4 g of water at 23.6 °c. the heat capacity of the empty calorimeter was 15.7 j/k. the final temperature of the calorimeter was 32.4 °c. what is the specific heat capacity of the mineral in j/gk?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 00:20, cmflores3245
4. propanol and isopropanol are isomers. this means that they have a) the same molecular formula but different chemical properties. b) different molecular formulas but the same chemical properties. c) the same molecular formula and the same chemical properties. d) the same molecular formula but represent different states of the compound
Answers: 3

Chemistry, 23.06.2019 06:00, BigGirlsTheBest
Amanda pushes a box across the room with a force of 30 n. it accelerates at 5 m/s/s. what is the mass of the box? * 6 kg 1.16 kg 30 kg 5kg
Answers: 2

Chemistry, 23.06.2019 09:00, floressavanna15
What properties would have caused early researchers to name hydrogen "inflammable air”
Answers: 3
Do you know the correct answer?
307 g of an unknown mineral is heated to 98.7 °c and placed into a calorimeter that contains 72.4 g...
Questions in other subjects:




Mathematics, 17.09.2019 00:00



Biology, 17.09.2019 00:00

Mathematics, 17.09.2019 00:00

Chemistry, 17.09.2019 00:00

Physics, 17.09.2019 00:00