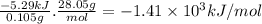Chemistry, 27.11.2019 19:31, billyeyelash
Abomb calorimeter has a heat capacity of 2.47 kj/k. when a 0.105-g sample of ethylene (c2h4) was burned in this calorimeter, the temperature increased by 2.14 k. calculate the energy of combustion for one mole of ethylene. a. –1.41 × 103 kj/mol b. –660 kj/mol c. –5.29 kj/mol d. –0.259 kj/mol e. –50.3 kj/mol

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, FloweyFlower
Aballoon inflated with three breaths of air has a volume of 1.7 l. at the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon? a balloon inflated with three breaths of air has a volume of 1.7 l. at the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon?
Answers: 3


Chemistry, 22.06.2019 18:50, cj31150631
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1

Chemistry, 22.06.2019 21:30, imalexiscv
Plzz a sample of table sugar (sucrose, c12h22o11) has a mass of 7.801 g. ● a) calculate the number of moles of c12h22o11 in the sample b) calculate the number of moles of each element in c12h22o11 (number of moles of c, number of moles of h & number of moles of o) in the sample. (use your answer from part a as your starting point.) show your work and highlight your final answer. calculate the number of atoms of each element in c12h22o11 (number of atoms of c, number of atoms of h & number of atoms of o) in the sample. (use your answers from part b as your starting for each element.) show your work and highlight your final answer.
Answers: 1
Do you know the correct answer?
Abomb calorimeter has a heat capacity of 2.47 kj/k. when a 0.105-g sample of ethylene (c2h4) was bur...
Questions in other subjects:



Mathematics, 30.10.2019 20:31