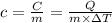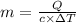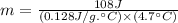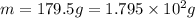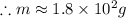Chemistry, 27.11.2019 19:31, jojo171717
If i apply 0.108 kj of energy in order to increase the temperature of a bar of gold from 30.0°c to 34.7°c, and the specific heat capacity of gold is 0.128 j/g°c, what is the mass the bar of gold in grams? a. 1.8 × 102 g b. 6.5 × 101 g c. 1.08 × 102 g d. 1.28 × 102 g e. none of these

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:30, I1YBYY
Infants born with severe respiratory problems are sometimes given liquid ventilation: they breathe a liquid that can dissolve more oxygen than air can hold. one of these liquids is a fluorinated compound, cf3(cf2)7br. the solubility of oxygen in this liquid is 66 mlo2 per 100 ml liquid. in contrast, air is 21 % oxygen by volume. calculate the moles of o2 present in an infant's lungs (volume: 12 ml ) if the infant takes a full breath of air. assume a pressure of 1 atm in the lungs.
Answers: 1

Chemistry, 21.06.2019 23:30, ashleyjaslin
Calculate the expected ph values of the buffer systems from the experiments (a, b,c, d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2


Chemistry, 22.06.2019 07:30, candigirl8847
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1
Do you know the correct answer?
If i apply 0.108 kj of energy in order to increase the temperature of a bar of gold from 30.0°c to 3...
Questions in other subjects:




Social Studies, 03.08.2019 18:30


Chemistry, 03.08.2019 18:30

Social Studies, 03.08.2019 18:30

Mathematics, 03.08.2019 18:30