
Chemistry, 27.11.2019 06:31, mdaniella522
2-phosphoglycerate(2pg) is converted to phosphoenolpyruvate (pep) by the enzyme enolase. the standard free energy change(deltago’) for this reaction is +1.7 kj/mol. if the cellular concentrations are 2pg = 0.5 mm and pep = 0.1 mm, what is the free energy change at 37 oc for the reaction 2pg ↔ pep? (a) 5.8 kj/mol(b) -5.8 kj/mol(c) +2.4 kj/mol(d) -2.4 kj/mol(e) -4146.4 kj/mol(f) +4146.4 kj/mol

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, anglacx5465
Why are pipes bursting in the in extremely cold weather?
Answers: 2



Chemistry, 23.06.2019 01:00, stefaniethibodeaux
Substance 33°f 100°f peanut oil solid liquid margarine solid liquid chocolate chips solid liquid which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
Do you know the correct answer?
2-phosphoglycerate(2pg) is converted to phosphoenolpyruvate (pep) by the enzyme enolase. the standar...
Questions in other subjects:




Chemistry, 18.12.2019 20:31




Mathematics, 18.12.2019 20:31



![Q_{r} =\frac{\left [ PEP \right ]}{\left [ 2PG \right ]} = \frac{0.1 mM}{0.5 mM} = 0.2](/tpl/images/0392/9257/2f0c6.png)
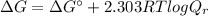
![\Delta G = 1.7 kJ/mol + [2.303 \times (8.314 \times 10^{-3} kJ/(K.mol))\times (310.15 K)] log (0.2)](/tpl/images/0392/9257/2a926.png)
![\Delta G = 1.7 + [5.938] \times (-0.699) = 1.7 - 4.15 = (-2.45 kJ/mol)](/tpl/images/0392/9257/65b39.png)




