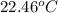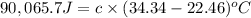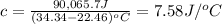Chemistry, 27.11.2019 06:31, lineaeriksen
A2.15g sample of benzene (c_6h_6) is burned in a bomb calorimeter, and the temperature rises from 22.46 degree c to 34.34 degree c. calculate the heat capacity of the bomb calorimeter. note the following thermochemical equation: c_6h_6(i) + 15/2 o_2 (g) rightarrow 6co_2 (g) + 3h_2o (g) delta h degree = -3267.5 kj

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:00, Isaiahtate053
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3

Chemistry, 22.06.2019 22:30, eduardoguizar8787
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1

Chemistry, 22.06.2019 23:30, shukriabdisabrie
Match each statement with the state of matter it describes
Answers: 3
Do you know the correct answer?
A2.15g sample of benzene (c_6h_6) is burned in a bomb calorimeter, and the temperature rises from 22...
Questions in other subjects:

Mathematics, 02.12.2021 20:20





Mathematics, 02.12.2021 20:20



English, 02.12.2021 20:20

Arts, 02.12.2021 20:20


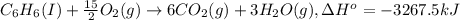
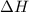 = enthalpy change = -3267.5 kJ/mol
= enthalpy change = -3267.5 kJ/mol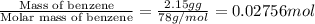
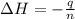
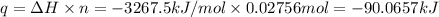
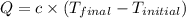
 = final temperature =
= final temperature = 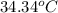
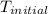 = initial temperature =
= initial temperature =