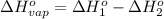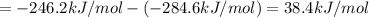Chemistry, 27.11.2019 03:31, KariSupreme
At a given set of conditions, 246.2 kj is given off when 1 mol of h2o(g) forms from its elements. under the same conditions, 284.6 kj is given off when 1 mol of h2o(l) forms from its elements. find δh for the vaporization of water at these conditions.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, officialgraciela67
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

Chemistry, 23.06.2019 00:00, sanaiajohnson56
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3


Chemistry, 23.06.2019 13:00, sports1997
Write the balanced chemical reaction for the formation of fe2(so4)3 from fe2o3 and so3 and determine how many moles of fe2(so4)3 are formed when 12.7 mol of so3 are reacted.
Answers: 1
Do you know the correct answer?
At a given set of conditions, 246.2 kj is given off when 1 mol of h2o(g) forms from its elements. un...
Questions in other subjects:


Biology, 20.07.2019 05:30

History, 20.07.2019 05:30

Health, 20.07.2019 05:30



Health, 20.07.2019 05:30

Mathematics, 20.07.2019 05:30



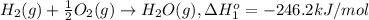 ..[1]
..[1]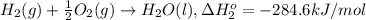 ..[2]
..[2]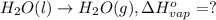 ...[3]
...[3]