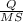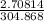If 50.00 ml of 1.05 m sodium hydroxide is added to 25.00 ml of 1.88 m hydrochloric acid, with both solutions originally at 24.66°c, what will be the final solution temperature? (assume that no heat is lost to the surrounding air and that the solution produced in the neutralization reaction has a density of 1.02 g/ml and a specific heat of 3.98 jg⁻¹°c⁻¹.)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:30, lizzyhearts
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

Chemistry, 22.06.2019 18:00, ambarpena14
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2


Chemistry, 22.06.2019 20:30, dinapaul424
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1
Do you know the correct answer?
If 50.00 ml of 1.05 m sodium hydroxide is added to 25.00 ml of 1.88 m hydrochloric acid, with both s...
Questions in other subjects:


Mathematics, 01.12.2020 01:00



Chemistry, 01.12.2020 01:00

Mathematics, 01.12.2020 01:00

Biology, 01.12.2020 01:00

Mathematics, 01.12.2020 01:00

Arts, 01.12.2020 01:00