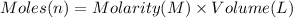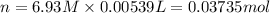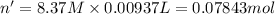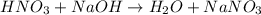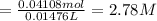Calculate the concentration of acid(or base) remaining in solution when 5.39ml of 6.93m hno3 is added to 9.37ml of 8.37m naoh. note the final volume is the sum of two added volumes. which of the following statement is true for the solution after mixing? a) naoh is in excess overhno3b)hno3 is in excess over naohc)hno3 and naoh are exactly balanced. what is the concentration of the excess naoh (or hno3) you indicated above?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:10, hunterthompson2
Which is true of transition metals when moving from left to right on the periodic table? the d sublevels are not filled across the period. the cation radii become larger across the period. atomic radii increase slightly and then start to decrease. atomic radii decrease slightly and then start to increase. o
Answers: 2

Chemistry, 22.06.2019 02:10, apowers6361
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3

Chemistry, 22.06.2019 06:30, rosieposie27
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 08:30, dyanaycooper13
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1
Do you know the correct answer?
Calculate the concentration of acid(or base) remaining in solution when 5.39ml of 6.93m hno3 is adde...
Questions in other subjects:


Physics, 31.07.2019 17:30


English, 31.07.2019 17:30

Mathematics, 31.07.2019 17:30


History, 31.07.2019 17:30

Chemistry, 31.07.2019 17:30



 .
.