

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:10, codeyhatch142
Nitric oxide (no) can be formed from nitrogen, hydrogen and oxygen in two steps. in the first step, nitrogen and hydrogen react to form ammonia: n2(g) + 2 h_2(g) rightarrow 2 nh_3 (g) delta h = -92. kj in the second step, ammonia and oxygen react to form nitric oxide and water: 4 nh_3(g) + 5 o_2(g) rightarrow 4no(g) + 6 h_2o(g) delta h = -905. kj calculate the net change in enthalpy for the formation of one mole of nitric oxide from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 1


Chemistry, 22.06.2019 04:00, soonerlady19
Which atom or ion is the largest? 0 a. 0 0 0 0 e. li
Answers: 2

Do you know the correct answer?
What is the ph of a solution prepared by mixing 50.00 ml of 0.10 m methylamine, ch3nh2, with 20.00 m...
Questions in other subjects:

Mathematics, 20.09.2020 05:01

English, 20.09.2020 05:01


English, 20.09.2020 05:01


Biology, 20.09.2020 05:01



Geography, 20.09.2020 05:01


![pOH=pKb+log\frac{[salt]}{[base]}](/tpl/images/0392/5893/d61df.png)
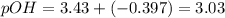
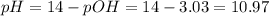
![[salt] = \frac{molarityXvolume}{finalvolume}=\frac{0.1X20}{(20+50)}= 0.0286M](/tpl/images/0392/5893/10d73.png)
![[base]= \frac{molarityXvolume}{finalvolume}=\frac{0.1X50}{(20+50)}= 0.0714M](/tpl/images/0392/5893/dea5f.png)
![3.43+log(\frac{[0.0286]}{0.0714}](/tpl/images/0392/5893/9272e.png)




