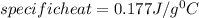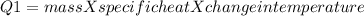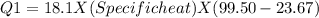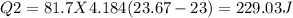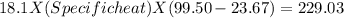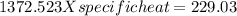Chemistry, 27.11.2019 01:31, sheram2010
In the laboratory, a student uses a coffee cup calorimeter to determine the specific heat of a metal. she heats 18.1 grams of lead to 99.50°c and then drops it into a cup containing 81.7 grams of water at 23.00°c. she measures the final temperature to be 23.67°c. assuming that all of the heat is transferred to the water, she calculates the specific heat of lead to be j/g°c.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:50, shonnybenskin8
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 05:50, ttangelique
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 07:30, nayiiii1874
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 19:00, Jasoncookies23
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1
Do you know the correct answer?
In the laboratory, a student uses a coffee cup calorimeter to determine the specific heat of a metal...
Questions in other subjects:


Mathematics, 24.10.2019 20:43


Mathematics, 24.10.2019 20:43