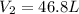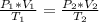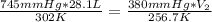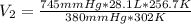Chemistry, 27.11.2019 00:31, happysage12
Aweather balloon is inflated to a volume of 28.1 l at a pressure of 745 mmhg and a temperature of 29.0 ∘c. the balloon rises in the atmosphere to an altitude where the pressure is 380. mmhg and the temperature is -16.3 ∘c. assuming the balloon can freely expand, calculate the volume of the balloon at this altitude.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, chrisxxxrv24
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1

Chemistry, 22.06.2019 04:00, kichensides
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1

Chemistry, 22.06.2019 12:30, kingbot350
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 13:50, amandamac7339
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1
Do you know the correct answer?
Aweather balloon is inflated to a volume of 28.1 l at a pressure of 745 mmhg and a temperature of 29...
Questions in other subjects:



Mathematics, 19.10.2021 05:40


Business, 19.10.2021 05:40


Chemistry, 19.10.2021 05:40