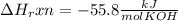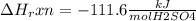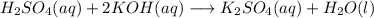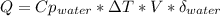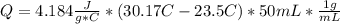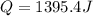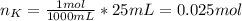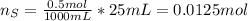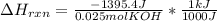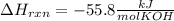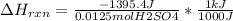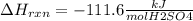When 25.0 ml of 0.500 m h2so4 is added to 25.00 mk of 1.00 m koh in a coffee-cup calorimeter at 23.50◦ c, the temperature rises to 30.17◦ c. calculate dhrxn for this reaction. (assume that the density of water of and specific heat capacity of the solution are the same as for pure water). ( –55.8 kj/mol koh, –112 kj/mol h2so4)

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:00, maryjane8872
Which set of characteristics best describes igneous rock? a) largest type of rock, made of organic matter, hardest type of rock b) least abundant type of rock, made of other rocks, made mostly of minerals c) found on all continents, contains wavy bands of stripes, contains fossils d) most abundant type in earth's crust, made of magma/lava, contains no fossils
Answers: 1

Chemistry, 22.06.2019 08:30, itzhari101
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

Chemistry, 22.06.2019 10:00, halohero7
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2
Do you know the correct answer?
When 25.0 ml of 0.500 m h2so4 is added to 25.00 mk of 1.00 m koh in a coffee-cup calorimeter at 23.5...
Questions in other subjects:







Mathematics, 19.07.2021 16:40