
Chemistry, 26.11.2019 23:31, sarahlearn3
Lithium and fluorine undergo ionic bonding. using the noble gas electron configurations for each (below), explain the process of bonding step by step, using proper grammar and mechanics. noble gas electron configurations: lithium: [he] 2s1 fluorine: [he] 2s22p5

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:40, btcastongia
Which is a difference between molecular compounds and ionic compounds? select the correct answer below: question 5 options: molecular compounds typically form between a metal and a nonmetal, while ionic compounds typically form between nonmetals. molecular compounds result from the transfer of electrons between atoms to form ions, while ionic compounds result from the sharing of electrons between neutral atoms. molecular compounds are formed of discrete, neutral molecules, while ionic compounds are formed of large repeating arrays of opposite charges. molecular compounds have high melting points and high boiling points, while ionic
Answers: 3

Chemistry, 22.06.2019 22:30, medinajocelyn45
Which compound most likely has the greatest bond energy?
Answers: 2

Chemistry, 23.06.2019 01:00, ZaNiyahlove4711
Which of the following is the molecular formula for a simple sugar? a. cooh b. h2o c. oh d. c6h12o6
Answers: 1

Chemistry, 23.06.2019 23:30, uliseshinojosa8
The current federal agency responsible for safety-related issues of nuclear energy is the: a - department of energy b - atomic energy commission c - nuclear regulatory commission d - environmental protection agency
Answers: 1
Do you know the correct answer?
Lithium and fluorine undergo ionic bonding. using the noble gas electron configurations for each (be...
Questions in other subjects:

Mathematics, 27.01.2021 09:40

Mathematics, 27.01.2021 09:40

History, 27.01.2021 09:40





Computers and Technology, 27.01.2021 09:40

Mathematics, 27.01.2021 09:40


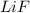 .
.![[He]2s^1](/tpl/images/0392/2650/6e198.png) .
.
![[He]2s^22p^5](/tpl/images/0392/2650/da8fe.png) .
.




