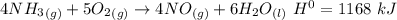Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:40, alexandraparava
For a patient with the following pes statement and interventions, which would be the most appropriate monitoring and evaluating data? pes statement: inadequate calcium intake related to food and nutrition related knowledge deficit as evidenced by statements that the only dietary source of calcium is milk and she believes that she is lactose intolerant. patient’s nutrition prescription is for a diet providing 1200 mg calcium per day. patient was provided with in-depth nutrition education on alternative dietary and supplement sources of calcium. a. calcium intake (at subsequent visit) b. knowledge assessment by asking patient to identify food sources from menus and shopping list (at the end of the current visit) c. serum calcium (at next visit) d. both a and b e. both a and c
Answers: 2

Chemistry, 22.06.2019 08:00, danielhall
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1

Chemistry, 23.06.2019 02:40, towelmearowel
Calculate the standard enthalpy of formation of liquid methanol, ch3oh(l), using the following information: c(graphite) + o2 latex: \longrightarrow ⟶ co2(g) latex: \delta δ h° = –393.5 kj/mol h2(g) + o2 latex: \longrightarrow ⟶ h2o(l) latex: \delta δ h° = –285.8 kj/mol ch3oh(l) + o2(g) latex: \longrightarrow ⟶ co2(g) + 2h2o(l) latex: \delta δ h° = –726.4 kj/mol
Answers: 3
Do you know the correct answer?
How much heat is absorbed/released when 35.00 g of nh3(g) reacts in the presence of excess o2(g) to...
Questions in other subjects:

Mathematics, 03.03.2021 22:10

History, 03.03.2021 22:10


Mathematics, 03.03.2021 22:10





Advanced Placement (AP), 03.03.2021 22:10


 :-
:-