Chemistry, 26.11.2019 21:31, Ciarrathereal
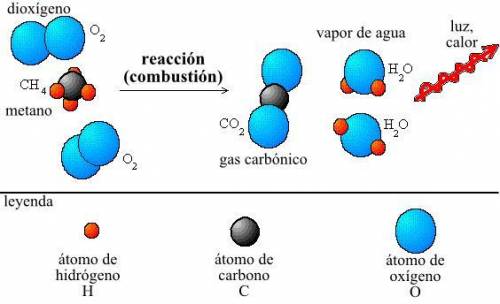
Achemist measures the energy change δh during the following reaction: c3h8 (g) +5o2 (g) →3co2 (g) +4h2o (l) =δh−2220.kj use the information to answer the following questions.
this reaction
endothermic.
exothermic.
suppose
81.0g
of
c3h8
react.
will any heat be released or absorbed?
yes, absorbed.
yes, released.
no.
if you said heat will be released or absorbed in the second part of this question, calculate how much heat will be released or absorbed.
kj
round your answer to
3
significant digits.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, dpazmembreno
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1

Chemistry, 22.06.2019 13:00, cnfndbxbfbdb2031
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2

Chemistry, 23.06.2019 03:50, arimarieestrada
How many liters of oxygen gas, at standardtemperature and pressure, will react with 35.8 grams ofiron metal? 4 fe (s) + 3 o2 (g) → 2 fe2o3 (s)
Answers: 3
Do you know the correct answer?
Achemist measures the energy change δh during the following reaction: c3h8 (g) +5o2 (g) →3co2 (g) +...
Questions in other subjects:

Mathematics, 23.04.2021 01:30



Computers and Technology, 23.04.2021 01:30




Mathematics, 23.04.2021 01:30

Mathematics, 23.04.2021 01:30

Mathematics, 23.04.2021 01:30


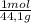 ×
×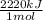 = 4,08x10³ kJ
= 4,08x10³ kJ