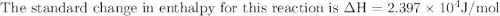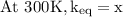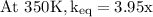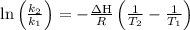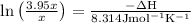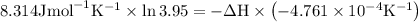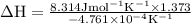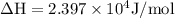Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, Luzperez09
Initially, the balloon had 3.0 liters of gas at a pressure of 400 kpa and was at a temperature of 294 k. if the balloon is cooled to 277 k and its volume decreased to 1 l, what will the new pressure in the balloon be?
Answers: 1

Chemistry, 21.06.2019 22:00, toledanomariap43bxm
Fission of uranium-235 products energy and a. isotopes of smaller elements b. isotopes of larger elements c. lighter isotopes of uranium d. heavier isotopes of uranium
Answers: 3

Chemistry, 22.06.2019 12:30, skaterwolf1317
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 23.06.2019 00:00, juliannasl
How is the way a mixture is combined different from how a compound is combined?
Answers: 3
Do you know the correct answer?
The equilibrium constant for a certain reaction increases by a factor of 3.95 when the temperature i...
Questions in other subjects:

English, 20.05.2021 22:10

Mathematics, 20.05.2021 22:10

Mathematics, 20.05.2021 22:10

Mathematics, 20.05.2021 22:10



Mathematics, 20.05.2021 22:10