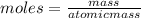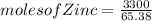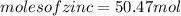Chemistry, 26.11.2019 07:31, loredohome
Galvanized nails are iron nails that have been plated with zinc to prevent rusting. the relevant reaction is
zn2+(aq)+2e−→zn(s)
for a large batch of nails, a manufacturer needs to plate a total zinc mass of 3.30 kg on the surface to get adequate coverage.
part a
how many moles of zinc are in 3.30 kg of zinc?
express your answer to three significant figures and include the appropriate units.
50.5 mol
submithintsmy answersgive upreview part
correct
significant figures feedback: your answer 50.47mol was either rounded differently or used a different number of significant figures than required for this part.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 00:30, portedon8644
13. calculate the initial concentration (before precipitation) of carbonate ions after the addition of each 0.05 ml of solution b to the 1.00 l beaker of solution a. divide the work among group members and write the answers in the table in model 3. assume the volume change as solution b is added is negligible. 14. notice the initial concentrations of zn2+ - and cu2+ in the table in model 3. a. explain how these were obtained from the data in model 2. b. as solution b is added and precipitates form, do these initial concentrations change? 15. use the data in model 2 to indicate the presence of precipitate (either znco3 or cuco3) after each 0.05 ml addition of solution b in model 3. 16. use the initial concentrations of carbonate ions and zinc ions to calculate the reaction quotient, qsp for the zinc carbonate scenarios in model 3. divide the work among group members and write the answers in the table in model 3. 17. use the initial concentrations of carbonate ion and copper(ii) ions to calculate the qsp for the copper(ii) carbonate scenarios in model 3. divide the work among group members and write the answers in the table in model 3.
Answers: 3


Chemistry, 22.06.2019 18:50, christhegreat1
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1
Do you know the correct answer?
Galvanized nails are iron nails that have been plated with zinc to prevent rusting. the relevant rea...
Questions in other subjects:









Mathematics, 21.06.2019 21:30

Mathematics, 21.06.2019 21:30