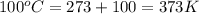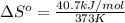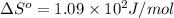Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, Riplilpeep
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1


Chemistry, 23.06.2019 01:30, kcarstensen59070
Which statement justifies that hydrogen peroxide (h2o2) is a polar molecule? the o – h bond is nonpolar and the molecule is asymmetric. the o – h bond is nonpolar and the molecule is symmetric. the o – h bond is polar and the molecule is asymmetric. the o – h bond is polar and the molecule is symmetric.
Answers: 1
Do you know the correct answer?
For water ∆h°vap = 40.7 kj/mol at 100.°c, its boiling point. calculate ∆s° for the vaporization of 1...
Questions in other subjects:


English, 26.06.2019 05:00



Mathematics, 26.06.2019 05:00

History, 26.06.2019 05:00

Mathematics, 26.06.2019 05:00

Arts, 26.06.2019 05:00


Mathematics, 26.06.2019 05:00


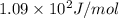
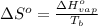
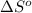 = change in entropy of vaporization = ?
= change in entropy of vaporization = ?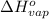 = change in enthalpy of vaporization = 40.7 kJ/mol
= change in enthalpy of vaporization = 40.7 kJ/mol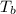 = boiling point temperature of water =
= boiling point temperature of water =