
Chemistry, 26.11.2019 06:31, maddiehope6893
The mole fraction of co2 in a certain solution with h2o as the solvent is 3.6 × 10−4. what is the approximate molality of co2 in this solution? a.0.00036 m. b.0.0065 m. c.0.020 m. d.2.0 × 10−5 m. e.6.5 m

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:50, kelonmazon2492
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 20:00, AaronEarlMerringer
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1

Chemistry, 22.06.2019 21:30, Turtlelover05
How can the periodic table be used to predict the behavior of elements?
Answers: 1
Do you know the correct answer?
The mole fraction of co2 in a certain solution with h2o as the solvent is 3.6 × 10−4. what is the ap...
Questions in other subjects:


English, 21.10.2020 17:01

Computers and Technology, 21.10.2020 17:01

Mathematics, 21.10.2020 17:01

Mathematics, 21.10.2020 17:01






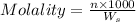
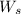 = weight of solvent in g
= weight of solvent in g  is =
is =  i.e.
i.e. =
= 






