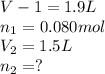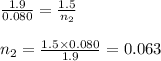Chemistry, 26.11.2019 06:31, jahootey2798
Aboy with pneumonia has lungs with a volume of 1.9 l that fill with 0.080 mol of air when he inhales. when he exhales, his lung volume decreases to 1.5 l. enter the number of moles of gas that remain in his lungs after he exhales. assume constant temperature and pressure. g

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, DarcieMATHlin2589
Write a brief passage describing a neutral atom of nitrogen-14 (n-14). describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. use the periodic table to you. 14 protons and eletrons since its a neutral atom
Answers: 1

Chemistry, 22.06.2019 08:30, mosthatedpicky1
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

Chemistry, 23.06.2019 06:30, destineedeal1
What type of chemical reaction occurs between silver nitrate (agno3) and copper (cu)? the equation i was given is 2agno3 + cu —> 2ag+ cu(no3)2.
Answers: 1
Do you know the correct answer?
Aboy with pneumonia has lungs with a volume of 1.9 l that fill with 0.080 mol of air when he inhales...
Questions in other subjects:

Biology, 30.08.2019 02:30

Health, 30.08.2019 02:30

Computers and Technology, 30.08.2019 02:30



History, 30.08.2019 02:30





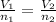
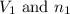 are the initial volume and number of moles
are the initial volume and number of moles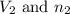 are the final volume and number of moles
are the final volume and number of moles