Chemistry, 26.11.2019 04:31, gracetay6873
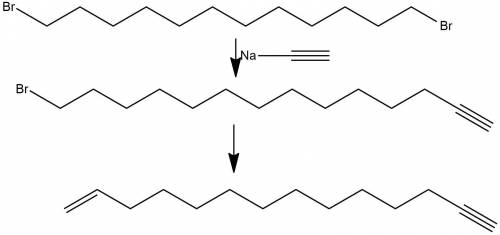
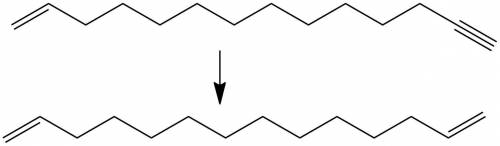
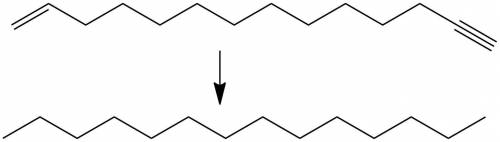
compound a has the molecular formula c14h25br and was obtained by reaction of sodium acetylide with 1,12-dibromododecane. on treatment of compound a with sodium amide, it was converted to compound b (c14h24). ozonolysis of compound b gave the compound with the following chemical formula: ho2c(ch2)12cooh (a diacid because it has two acid functional groups on each end). catalytic hydrogenation of compound b over lindar palladium gave compound c (c14h26) and hydrogenation of compound b over platinum gave compound d (c14h28). sodium-ammonia reaction of compound b gave compound e (c14h26). both c and e yielded o=ch(ch2)12ch=o on ozonolysis. assign structures to compounds a through e so as to be consistent with the observed transformations.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, palcochran1313
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1


Chemistry, 22.06.2019 17:20, phanuel642
The small bags of silica gel you often see in a new shoe box are placed there to control humidity. despite its name, silica gel is a solid. it is a chemically inert, highly porous, amorphous form of sio2. because water vapor readily adsorbs onto the surface of silica gel, it acts as a desiccant. despite not knowing mechanistic details of the adsorption of water onto silica gel, from the information provided you should be able to make an educated guess about the thermodynamic characteristics of the process. predict the signs for δg, δh, and δs for the adsorption of water.
Answers: 2

Chemistry, 22.06.2019 18:00, kyllow5644
Answer asap need to be answered by wednesday morning explain how a buffer works, using an ethanoic acid / sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 3
Do you know the correct answer?
compound a has the molecular formula c14h25br and was obtained by reaction of sodium acetylide with...
Questions in other subjects:

Mathematics, 16.10.2020 03:01

Medicine, 16.10.2020 03:01



Physics, 16.10.2020 03:01


Mathematics, 16.10.2020 03:01


Mathematics, 16.10.2020 03:01

Mathematics, 16.10.2020 03:01