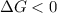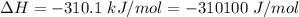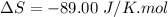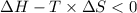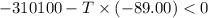Chemistry, 26.11.2019 03:31, Rileyb101207
Areaction will be spontaneous only at low temperatures if both δh and δs are negative. for a reaction in which δh = −310.1 kj/mol and δs = −89.00 j/k · mol, determine the temperature (in °c) below which the reaction is spontaneous.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, isalih7256
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1

Chemistry, 22.06.2019 10:30, angemango3423
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1


Chemistry, 22.06.2019 12:00, carvajalj2520
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1
Do you know the correct answer?
Areaction will be spontaneous only at low temperatures if both δh and δs are negative. for a reactio...
Questions in other subjects:


Mathematics, 26.11.2019 03:31

Mathematics, 26.11.2019 03:31

History, 26.11.2019 03:31



Biology, 26.11.2019 03:31


Mathematics, 26.11.2019 03:31


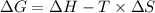
 is the change in the Gibbs free energy.
is the change in the Gibbs free energy.
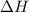 is the enthalpy change of the reaction.
is the enthalpy change of the reaction.
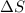 is the change in entropy.
is the change in entropy.