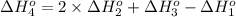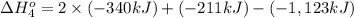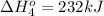Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:20, anggar20
Why does the sun appear to be the brightest star in the sky? a- its apparent brightness is much greater than other stars. b- it burns more gas, making it brighter than any other star. c- it is the largest star in the galaxy, so it is the brightest star. d- its relative distance to earth is closer than the other stars.
Answers: 1


Chemistry, 22.06.2019 20:20, Matseleng3775
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1
Do you know the correct answer?
Calculate the enthalpy for this reaction: 2c(s) + h2(g) > c2h2(g) δh° = kj given the following...
Questions in other subjects:


Mathematics, 22.06.2019 20:30


Mathematics, 22.06.2019 20:30

Mathematics, 22.06.2019 20:30

Biology, 22.06.2019 20:30


English, 22.06.2019 20:30



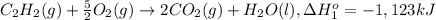 ...[1]
...[1]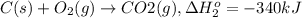 ..[2]
..[2]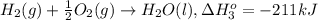 ..[3]
..[3]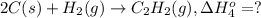 ..[4]
..[4]