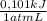Chemistry, 26.11.2019 03:31, shelbylynn737
Find δe° for the reaction below if the process is carried out at a constant pressure of 1.00 atm andδv (the volume change) = -24.5 l. (1 l ∙ atm = 101 j)
2 co(g) + o2 (g) → 2 co2(g) δh° = -566. kj
a. +2.47 kj
b. -568 kj
c. -2.47 kj
d. -564 kj

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:40, gabrielolivas59
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 15:00, raeprince9213
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 22.06.2019 15:20, mydoggy152
Fossil fuels are organic compounds that are made from
Answers: 1

Chemistry, 22.06.2019 15:50, Edwardwall
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1
Do you know the correct answer?
Find δe° for the reaction below if the process is carried out at a constant pressure of 1.00 atm and...
Questions in other subjects:

Mathematics, 25.10.2020 14:00





Mathematics, 25.10.2020 14:00



Arts, 25.10.2020 14:00

Mathematics, 25.10.2020 14:00