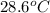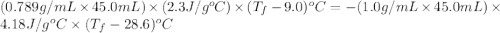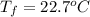Chemistry, 26.11.2019 02:31, kylemartinez13
If 45.0 ml of ethanol (density=0.789 g/ml) initially at 9.0 c is mixed with 45.0 ml of water (density=1.0 g/ml) initially at 28.6 c in an insulated beaker, and assuming that no heat is lost, what is the final temperature of the mixture?
i tried many times in trying to get the answer, but i keep getting it wrong. i appreciate who answers this writes it step by step. you.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, laurachealsy923
In an energy pyramid, which level has the most available energy?
Answers: 1

Chemistry, 22.06.2019 07:30, veronica25681
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1

Chemistry, 22.06.2019 11:00, hannah5143
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2

Chemistry, 22.06.2019 16:10, nauticatyson9
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1
Do you know the correct answer?
If 45.0 ml of ethanol (density=0.789 g/ml) initially at 9.0 c is mixed with 45.0 ml of water (densit...
Questions in other subjects:




Mathematics, 30.09.2021 23:40

English, 30.09.2021 23:40


Biology, 30.09.2021 23:40

Mathematics, 30.09.2021 23:40

History, 30.09.2021 23:40


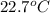
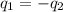
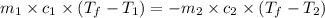
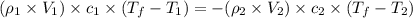
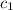 = specific heat of ethanol =
= specific heat of ethanol = 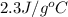
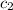 = specific heat of water =
= specific heat of water = 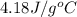
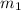 = mass of ethanol
= mass of ethanol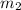 = mass of water
= mass of water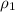 = density of ethanol = 0.789 g/mL
= density of ethanol = 0.789 g/mL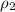 = density of water = 1.0 g/mL
= density of water = 1.0 g/mL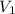 = volume of ethanol = 45.0 mL
= volume of ethanol = 45.0 mL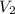 = volume of water = 45.0 mL
= volume of water = 45.0 mL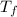 = final temperature of mixture = ?
= final temperature of mixture = ?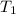 = initial temperature of ethanol =
= initial temperature of ethanol = 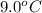
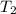 = initial temperature of water =
= initial temperature of water =