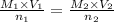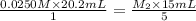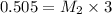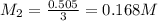Ferris & mona used the orp sensor to titrate a ferrous ammonium sulfate solution, (nh4)2fe(so4)2 with kmno4 titrant.
they titrated a 15.00 ml aliquot of the fe+2 solution with 0.0250 m mno4- solution and determined that the equivalence point was at 20.2 ml.
what is the molarity of the fe+2 solution? 5 fe+2(aq) + mno4-(aq) + 8 h+(aq) â 5 fe+3(aq) + mn+2(aq) + 4 h2oselect one: a. 0.168 mb. 0.0928 mc. 0.0337 md. 0.673 m

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 23:30, tylerineedhelp
Ihat will happen if i added baking soda to vinegar
Answers: 2

Chemistry, 22.06.2019 10:00, JOEFRESH10
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

Chemistry, 22.06.2019 12:50, khorasanpublic
The number at the end of an isotope’s name is the number.
Answers: 1
Do you know the correct answer?
Ferris & mona used the orp sensor to titrate a ferrous ammonium sulfate solution, (nh4)2fe(so4)...
Questions in other subjects:

Mathematics, 13.12.2021 01:00

History, 13.12.2021 01:00

Mathematics, 13.12.2021 01:00

English, 13.12.2021 01:00

History, 13.12.2021 01:00

Mathematics, 13.12.2021 01:00

Mathematics, 13.12.2021 01:00