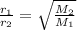Chemistry, 26.11.2019 01:31, jeovontamarley
The rate of effusion of a gas, r, is inversely proportional to the square root of its molar mass, m. the relative rate of two different gases is expressed as r1r2=m2m1−−−√ where r1 and r2 are the effusion rates of two gases and m1 and m2 are their respective molar masses. part a in an effusion experiment, it was determined that nitrogen gas, n2, effused at a rate 1.812 times faster than an unknown gas. what is the molar mass of the unknown gas? express your answer to four significant figures and include the appropriate units.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:10, amuijakobp78deg
Agas mixture with a total pressure of 745 mmhg contains each of the following gases at the indicated partial pressures: co2, 245 mmhg ; ar, 119 mmhg ; and o2, 163 mmhg . the mixture also contains helium gas. part a what is the partial pressure of the helium gas? phe p h e = nothing mmhg request answer part b what mass of helium gas is present in a 10.2-l sample of this mixture at 283 k ? m m = nothing g request answer
Answers: 1


Chemistry, 21.06.2019 21:00, tamikagoss22
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 22.06.2019 02:10, fvmousdiana
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2
Do you know the correct answer?
The rate of effusion of a gas, r, is inversely proportional to the square root of its molar mass, m....
Questions in other subjects:


Biology, 05.11.2020 20:50

Mathematics, 05.11.2020 20:50

English, 05.11.2020 20:50

Chemistry, 05.11.2020 20:50


History, 05.11.2020 20:50



History, 05.11.2020 20:50