Chemistry, 26.11.2019 01:31, billlyyyyyyyyyy
Calculate the change internal energy (δe) for a system that is giving off 65.0 kj of heat and is performing 855 j of work on the surroundings. calculate the change internal energy (δe) for a system that is giving off 65.0 kj of heat and is performing 855 j of work on the surroundings. 9.00 x 102 kj 64.1 kj -9.00 x 102 kj -64.1 kj -65.9 kj

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 23.06.2019 00:30, vane6176
You are attempting to recrystallize a crude product mixture. you add the appropriate amount of hot solvent and are allowing the solution to slowly cool to room temperature. however, at room temperature no crystals have appeared, which of the following methods should be used to induce crystallization? choose all correct answers. a) place the flask in an ice bath. b) swirl the contents of the flask. c) add a small seed crystal of the desired product. d) scratch the inside of the glassware using a stir rod. it can be multiple choices
Answers: 3
Do you know the correct answer?
Calculate the change internal energy (δe) for a system that is giving off 65.0 kj of heat and is per...
Questions in other subjects:





Chemistry, 07.10.2019 02:30

Mathematics, 07.10.2019 02:30

Mathematics, 07.10.2019 02:30


Mathematics, 07.10.2019 02:30


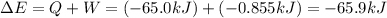
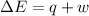
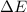 =Change in internal energy
=Change in internal energy
 {Work is done by the system is negative as the final volume is greater than initial volume}
{Work is done by the system is negative as the final volume is greater than initial volume}