
Chemistry, 26.11.2019 00:31, IzzybellaRamilo
The reaction 2no(g)+o2(g)−→−2no2(g) is second order in no and first order in o2. when [no]=0.040m, and [o2]=0.035m, the observed rate of disappearance of no is 9.3×10−5m/s.
(a) what is the rate of disappearance of o2 at this moment?
(b) what is the value of the rate constant?
(c) what are the units of the rate constant?
(d) what would happen to the rate if the concentration of no were increased by a factor of 1.8?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:10, vapelordcarl69
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3


Chemistry, 22.06.2019 15:00, tcapele252
‘which reaction would most likely require the use of an inert electrode?
Answers: 1
Do you know the correct answer?
The reaction 2no(g)+o2(g)−→−2no2(g) is second order in no and first order in o2. when [no]=0.040m, a...
Questions in other subjects:

Mathematics, 11.09.2020 17:01

Mathematics, 11.09.2020 17:01

English, 11.09.2020 17:01

Mathematics, 11.09.2020 17:01

Mathematics, 11.09.2020 17:01

Physics, 11.09.2020 17:01

Mathematics, 11.09.2020 17:01

Mathematics, 11.09.2020 17:01

Mathematics, 11.09.2020 17:01

History, 11.09.2020 17:01


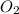 is:
is:  M/s
M/s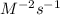
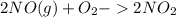
![-\frac{1}{2} \frac{d}{dt}[NO]](/tpl/images/0390/5846/9a676.png) =
= ![-\frac{d}{dt}[O_{2}]](/tpl/images/0390/5846/2117c.png) =
= ![\frac{1}{2}\frac{d}{dt}[NO_{2}]](/tpl/images/0390/5846/27827.png) -----(1)
-----(1)![[NO]^{2}[O_{2}]](/tpl/images/0390/5846/d8f06.png) -----(2)
-----(2)![-\frac{d}{dt}[NO]](/tpl/images/0390/5846/badcd.png) =
= 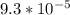 M/s.
M/s.![\frac{rate}{[NO]^{2}[O_{2}]}](/tpl/images/0390/5846/0588c.png)
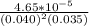 = 0.83036
= 0.83036 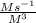 =
= ![rate\alpha [NO]^{2}](/tpl/images/0390/5846/ed652.png)
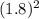 = 3.24
= 3.24



