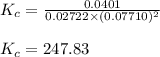Chemistry, 26.11.2019 00:31, bella122805
Amixture of gaseous co and h2, called synthesis gas, is used commercially to prepare methanol (ch3oh), a compound considered an alternative fuel to gasoline. under equilibrium conditions at 550.3 k, [h2] = 0.07710 mol/l, [co] = 0.02722 mol/l, and [ch3oh] = 0.0401 mol/l. what is the value of kc for this reaction at 550.3 k?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:50, Jerrikasmith28
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 04:30, rosetoheart2
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1

Chemistry, 22.06.2019 06:00, applejulianamoreno
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3

Chemistry, 22.06.2019 12:30, quantamagic
Word equation for k(s)+h2o(l) yield koh(aq) + h2(g)
Answers: 1
Do you know the correct answer?
Amixture of gaseous co and h2, called synthesis gas, is used commercially to prepare methanol (ch3oh...
Questions in other subjects:


History, 07.04.2020 05:48





Biology, 07.04.2020 05:48




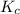 for the reaction at 550.3 K is 247.83
for the reaction at 550.3 K is 247.83
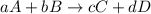
![K_{c}=\frac{[C]^c[D]^d}{[A]^a[B]^b}](/tpl/images/0390/5865/b6f47.png)
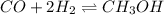
![K_c=\frac{[CH_3OH]}{[CO][H_2]^2}](/tpl/images/0390/5865/4cf94.png)
![[CH_3OH]=0.0401mol/L](/tpl/images/0390/5865/4486b.png)
![[CO]=0.02722mol/L](/tpl/images/0390/5865/ab367.png)
![[H_2]=0.07710mol/L](/tpl/images/0390/5865/326e6.png)