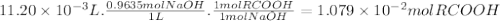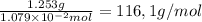Chemistry, 25.11.2019 23:31, golffuture666
What is the molecular weight of a monoprotic carboxylic acid if 11.20 ml of 0.9635 m sodium hydroxide is required to titrate 1.253 g of this acid? the reaction is represented by following equation. note: in the equation, r represents an unspecified carbon containing structure rco-h (aq)naoh (aq) rco, na (aq) hoh (u) separate experiments suggest the unknown acid is likely pentanoic acid, c4h, co2h. is the unknown pentanoic acid? why or why not?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:50, kelonmazon2492
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3


Chemistry, 23.06.2019 04:20, lelliott86
The lewis diagrams for magnesium and fluorine are shown below. what is the correct chemical formula for magnesium fluoride? a. mgf b. mg2f c. mgf2 d. mg2f3
Answers: 1

Chemistry, 23.06.2019 07:40, wrestling2
What is the reduction potential of a hydrogen electrode that is still at standard pressure, but has ph = 5.65 , relative to the she?
Answers: 1
Do you know the correct answer?
What is the molecular weight of a monoprotic carboxylic acid if 11.20 ml of 0.9635 m sodium hydroxid...
Questions in other subjects:



Advanced Placement (AP), 18.12.2020 01:00


Mathematics, 18.12.2020 01:00

Computers and Technology, 18.12.2020 01:00

History, 18.12.2020 01:00



Mathematics, 18.12.2020 01:00