Chemistry, 25.11.2019 23:31, ZeroFrost7899
Calculate δh∘f (in kilojoules per mole) for benzene, c6h6, from the following data: 2c6h6(l) + 15o2(g)→12co2(g)+6h2o(l) δh∘=−6534.0 kjδh∘f co2=−393.5 kj/molδh∘f h2o=−285.8 kj/mol express the enthalpy change in kilojoules per mole to three significant digits.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, webbhlharryteach
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 05:30, greekfreekisdbz
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3


Chemistry, 23.06.2019 03:00, draveon6925
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers: 2
Do you know the correct answer?
Calculate δh∘f (in kilojoules per mole) for benzene, c6h6, from the following data: 2c6h6(l) + 15o2...
Questions in other subjects:

Biology, 22.04.2020 00:38

Mathematics, 22.04.2020 00:38



Social Studies, 22.04.2020 00:38






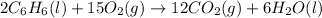
![\Delta H=\sum [n\times \Delta H_f(product)]-\sum [n\times \Delta H_f(reactant)]](/tpl/images/0390/4702/76c37.png)
![\Delta H=[(n_{CO_2}\times \Delta H_{CO_2})+(n_{H_2O}\times \Delta H_{H_2O})]-[(n_{O_2}\times \Delta H_{O_2})+(n_{C_6H_6}\times \Delta H_{C_6H_6})]](/tpl/images/0390/4702/15aa8.png)
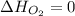 (as heat of formation of substances in their standard state is zero
(as heat of formation of substances in their standard state is zero![-6534.0=[(12\times -393.5)+(6\times -285.8)]-[(15\times 0)+(2\times \Delta H_{C_6H_6})]](/tpl/images/0390/4702/ed6d2.png)