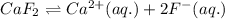Ionic solids dissolve in water and break up into their ions. however, some ionic solids only partially dissolve, leaving a significant amount of solid undissolved. in some cases, the amount that dissolves is very small and is almost zero.
a mathematical formula that indicates the extent to which an ionic solid dissolves in water is called the solubility product constant. the solubility product constant, commonly referred to as ksp, indicates the extent to which an ionic solid dissolves.
which of the following is the correct solubility product constant for the reaction shown below?
caf₂ ⇌ ca²⁺(aq) + 2f⁻
a. ksp = [ca²⁺][f⁻]
b. ksp = [ca²⁺]2[f⁻]
c. ksp = [ca²⁺]2[f⁻]²
d. ksp = [ca²⁺][f⁻]²

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:10, chloeholt123
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 15:00, raeprince9213
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 22.06.2019 19:40, trodgers0202
Scientists have developed an explanation of a phenomenon from several verified hypotheses. the explanation has been confirmed through numerous experimental tests. which option best describes this explanation? a. scientific lawb. research questionc. hypothesisd. scientific theory
Answers: 3

Chemistry, 22.06.2019 21:30, imalexiscv
Plzz a sample of table sugar (sucrose, c12h22o11) has a mass of 7.801 g. ● a) calculate the number of moles of c12h22o11 in the sample b) calculate the number of moles of each element in c12h22o11 (number of moles of c, number of moles of h & number of moles of o) in the sample. (use your answer from part a as your starting point.) show your work and highlight your final answer. calculate the number of atoms of each element in c12h22o11 (number of atoms of c, number of atoms of h & number of atoms of o) in the sample. (use your answers from part b as your starting for each element.) show your work and highlight your final answer.
Answers: 1
Do you know the correct answer?
Ionic solids dissolve in water and break up into their ions. however, some ionic solids only partial...
Questions in other subjects:





English, 17.03.2020 01:25


History, 17.03.2020 01:25

Mathematics, 17.03.2020 01:25



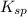 for calcium fluoride is
for calcium fluoride is ![K_{sp}=[Ca^{2+}][2F^-]^2](/tpl/images/0390/3792/f6d9c.png)