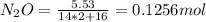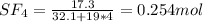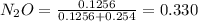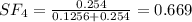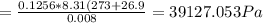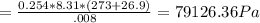A8.00 l tank at 26.9 c is filled with 5.53 g of dinitrogen difluoride gas and 17.3 g of sulfur hexafluoride gas. you can assume both gases behave as ideal gases under these conditions. calculate the mole fraction and partial pressure of each gas, and the total pressure in the tank

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, smartboy2296
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 21:30, imalexiscv
Plzz a sample of table sugar (sucrose, c12h22o11) has a mass of 7.801 g. ● a) calculate the number of moles of c12h22o11 in the sample b) calculate the number of moles of each element in c12h22o11 (number of moles of c, number of moles of h & number of moles of o) in the sample. (use your answer from part a as your starting point.) show your work and highlight your final answer. calculate the number of atoms of each element in c12h22o11 (number of atoms of c, number of atoms of h & number of atoms of o) in the sample. (use your answers from part b as your starting for each element.) show your work and highlight your final answer.
Answers: 1

Chemistry, 23.06.2019 03:00, makayyafreeman
Select the correct answer. wax is a nonpolar substance. in which type of substance is it the most soluble?
Answers: 1

Chemistry, 23.06.2019 07:30, 22emilyl530
Chris is about to do an experiment to measure the density of water at several temperatures. his teacher has him prepare and sign a safety contract before beginning the experiment. which term is mostlikely part of the safety contract
Answers: 3
Do you know the correct answer?
A8.00 l tank at 26.9 c is filled with 5.53 g of dinitrogen difluoride gas and 17.3 g of sulfur hexaf...
Questions in other subjects:

Mathematics, 12.03.2021 22:40

Mathematics, 12.03.2021 22:40

Mathematics, 12.03.2021 22:40

Mathematics, 12.03.2021 22:40

History, 12.03.2021 22:40




Social Studies, 12.03.2021 22:40