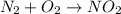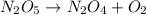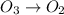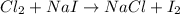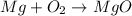Be sure to answer all parts. write an unbalanced equation to represent each of the following reactions: do not include phase abbreviations. (a) nitrogen and oxygen react to form nitrogen dioxide. (b) dinitrogen pentoxide reacts to form dinitrogen tetroxide and oxygen. (c) ozone reacts to form oxygen. (d) chlorine and sodium iodide react to form iodine and sodium chloride. (e) magnesium and oxygen react to form magnesium oxide.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, willcohen42
150.0 grams of asf3 were reacted with 180.0 g of ccl4 to produce ascl3 and ccl2f2. if the actual yield of ccl2f2 was 127 g, what is the percent yield?
Answers: 2

Chemistry, 21.06.2019 22:30, kiki197701
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3

Chemistry, 22.06.2019 07:30, bbyniah123
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

Chemistry, 23.06.2019 00:00, baseball1525
Which item is most likely part of the safety contract
Answers: 1
Do you know the correct answer?
Be sure to answer all parts. write an unbalanced equation to represent each of the following reactio...
Questions in other subjects:


History, 31.01.2022 17:20

English, 31.01.2022 17:20

Chemistry, 31.01.2022 17:20




Chemistry, 31.01.2022 17:30


Mathematics, 31.01.2022 17:30