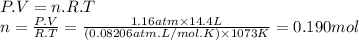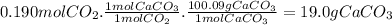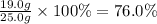Chemistry, 25.11.2019 21:31, zanaplen27
For the reaction below, kp = 1.16 at 800.°c. caco3(s) equilibrium reaction arrow cao(s) + co2(g) if a 25.0-g sample of caco3 is put into a 14.4 l container and heated to 800°c, what percentage by mass of the caco3 will react to reach equilibrium?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:00, claudia122752
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1


Chemistry, 22.06.2019 20:00, teacherpreacher
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2
Do you know the correct answer?
For the reaction below, kp = 1.16 at 800.°c. caco3(s) equilibrium reaction arrow cao(s) + co2(g) if...
Questions in other subjects:


Social Studies, 04.10.2019 23:40

Chemistry, 04.10.2019 23:40

Chemistry, 04.10.2019 23:50



English, 04.10.2019 23:50