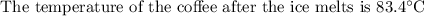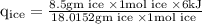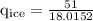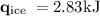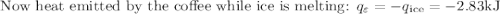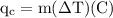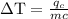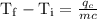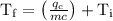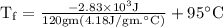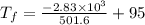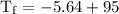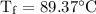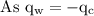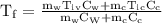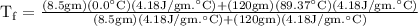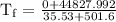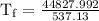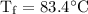Chemistry, 25.11.2019 21:31, jenorajordan5387
An ice cube of mass 8.5g is added to a cup of coffee, whose temperature is 95 degrees celcius and which contains 120 g of liquid. assume the specific heat capacity of coffee is the same as that of water. the heat of fusion of the ice (the heat associated with ice melting) is 6.0 kj/mol. find the temperature of the coffee after the ice melts.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:00, uniqueray33
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 22.06.2019 10:30, kylemartinez13
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Chemistry, 22.06.2019 10:30, Brookwiggington8814
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1
Do you know the correct answer?
An ice cube of mass 8.5g is added to a cup of coffee, whose temperature is 95 degrees celcius and wh...
Questions in other subjects:


History, 17.07.2019 00:30


Chemistry, 17.07.2019 00:30

Mathematics, 17.07.2019 00:30