
Chemistry, 25.11.2019 20:31, staceyfrog2002ovw02g
Combustion of hydrocarbons such as butane () produces carbon dioxide, a "greenhouse gas." greenhouse gases in the earth's atmosphere can trap the sun's heat, raising the average temperature of the earth. for this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide. write a balanced chemical equation, including physical state symbols, for the combustion of gaseous butane into gaseous carbon dioxide and gaseous water. 2. suppose 0.360 kg of butane are burned in air at a pressure of exactly 1 atm and a temperature of 20.0 °c. calculate the volume of carbon dioxide gas that is produced be sure your answer has the correct number of significant digits.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:00, applejulianamoreno
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3

Chemistry, 23.06.2019 01:00, jazzy200232
Which process results in the release of energy stored in the products of photosynthesis? a. polymer synthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1

Chemistry, 23.06.2019 02:00, FailingstudentXD
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water
Answers: 1
Do you know the correct answer?
Combustion of hydrocarbons such as butane () produces carbon dioxide, a "greenhouse gas." greenhouse...
Questions in other subjects:



History, 12.12.2020 16:40


Mathematics, 12.12.2020 16:40

English, 12.12.2020 16:40



Computers and Technology, 12.12.2020 16:40


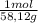 =6,19moles of butane
=6,19moles of butane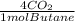 = 24,8 moles CO₂
= 24,8 moles CO₂



