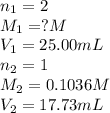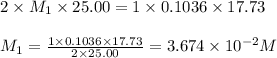Chemistry, 25.11.2019 19:31, michellectucker1982
Astudent titrated a 25.00-ml sample of a solution containing an unknown weak, diprotic acid (h2a) with n2oh. if the titration required 17.73 ml of 0.1036 m n2oh to completely neutralize the acid, calculate the concentration (in m) of the weak acid in the sample.
(a) 9.184 x 10 m
(b) 3.674 x 10-2 m
(c) 7.304 x 10-2 m
(d) 7.347 x 10-2 m
(e) 1.469 x 101 m

Answers: 3
Other questions on the subject: Chemistry




Chemistry, 22.06.2019 20:30, ashley4329
Select all the correct answers. which compounds have the empirical formula ch20? (multiple answers)a. c2h4o2b. c3h603c. ch2o2d. c5h1005e. c6h1206
Answers: 2
Do you know the correct answer?
Astudent titrated a 25.00-ml sample of a solution containing an unknown weak, diprotic acid (h2a) wi...
Questions in other subjects:

Mathematics, 22.06.2021 18:10

Mathematics, 22.06.2021 18:10









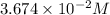
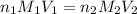
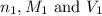 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 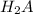
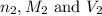 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.