Answers: 2
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 18:10, write2lakenor7awj
Which statements describe polyatomic ions? check all that apply. polyatomic ions have many charges. polyatomic ions have one overall charge. polyatomic ions repel other ions to form ionic bonds. polyatomic ions attract other ions to form ionic bonds. polyatomic ions are made up of only one type of atom. polyatomic ions are made up of two or more types of atoms.
Answers: 2


Chemistry, 22.06.2019 02:50, giiffnlojd
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3
Do you know the correct answer?
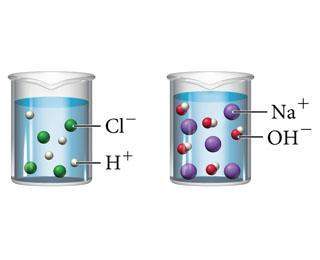
These two beakers represent solutions of hcl and naoh. describe a third beaker showing the ions that...
Questions in other subjects:

Mathematics, 24.09.2021 16:00



Mathematics, 24.09.2021 16:00

Mathematics, 24.09.2021 16:00


Business, 24.09.2021 16:00

Mathematics, 24.09.2021 16:00

Mathematics, 24.09.2021 16:00