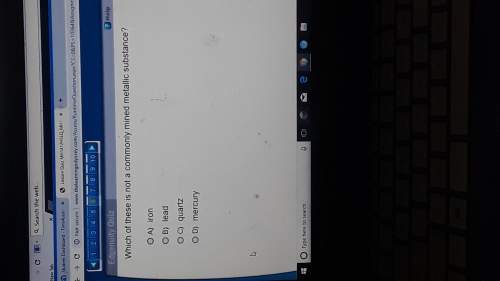
Chemistry, 24.11.2019 11:31, hannah2757
Al2o3 + 6 hcl ↔ 2 alcl3 + 3 h2o if you start with 67.7 grams al2o3 and 196.8 grams hcl, how many grams of alcl3 can be produced by the reaction?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, bettybales1986
According to the tide table below what time of day will the highest tide occur?
Answers: 1

Chemistry, 22.06.2019 06:30, yolo123321
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 22.06.2019 12:10, yootmytoot
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution. calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 22.06.2019 12:10, coastieltp58aeg
Building glycogen from glucose molecules is an example of
Answers: 3
Do you know the correct answer?
Al2o3 + 6 hcl ↔ 2 alcl3 + 3 h2o if you start with 67.7 grams al2o3 and 196.8 grams hcl, how many gra...
Questions in other subjects:




English, 22.06.2019 09:30




Mathematics, 22.06.2019 09:30


Mathematics, 22.06.2019 09:30